- 2025
-
Ruthenium-Catalyzed Enyne Metathesis: An Entry to Functionalized Azaborine Heterocycles
K. Mao, F. Fontaine-Vive, R. Melot, Véronique Michelet, Org. Lett. 2025 (ASAP)

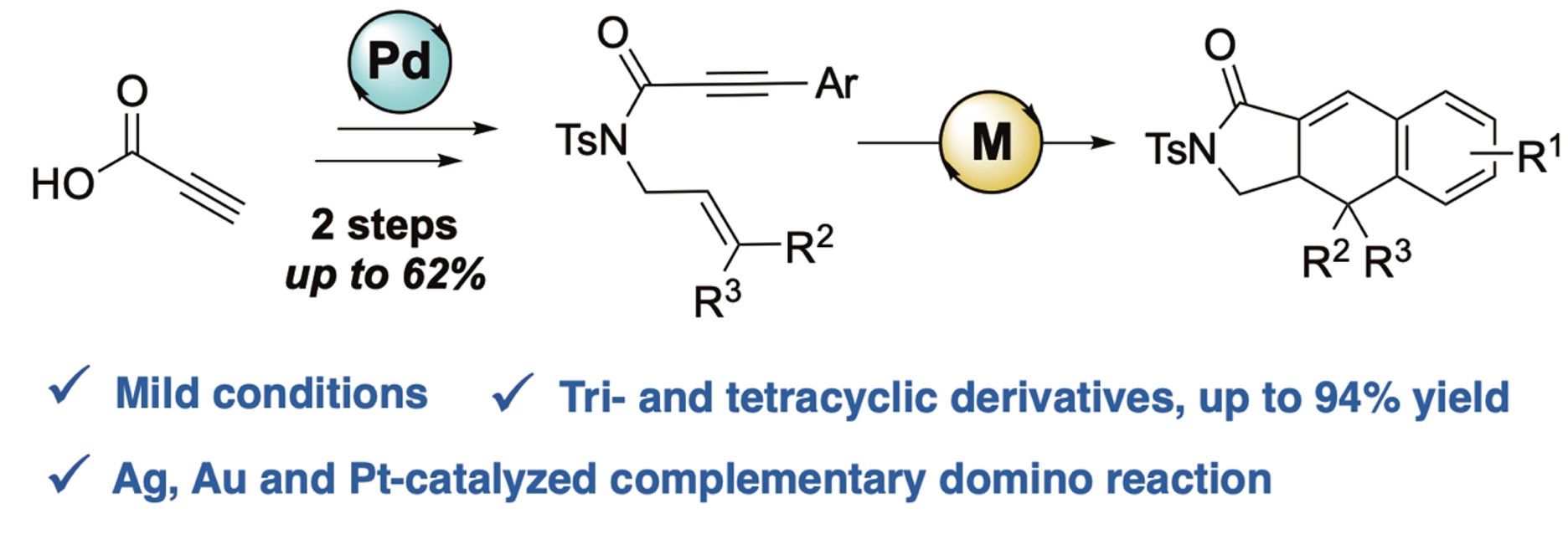
Silver-, gold-, and platinum-catalyzed [4+2] cyclizations: synthesis of 2,3,3a,4-tetrahydro-1H-benzo[f]isoindol-1-one derivatives (Special issue : Women Chemists in France in 2025)
K. Mao, K. Plevova, X. Chen, L. J. Prieto Pabon, S. Poulain-Martini, V. Michelet, Comptes Rendus. Chimie 2025, 28, 53-59
- 2024
-
Small gold nanoparticles for tandem cyclization/reduction and cyclization/hydroalkoxylation reactions
K. Plevová, V. Michelet, S. Antoniotti, Commun. Chem. 2024, 7, 1-9
Gold(I)-Catalyzed Functionalization of Pyrrole Derivatives: Stereoselective Synthesis of 7,8-Disubstituted 7,8-Dihydroindolizin-6(5H)-ones
A. Truchon, M. Gorodnichy, S. Olivero, V. Michelet, Org. Lett. 2024, 26, 6698–6702

Au- and Pd-catalyzed cyclization processes: synthesis of polyfunctionalized cyclopropanes
A. Truchon, A. Dupeux, S. Olivero, V. Michelet, Org. Chem. Front., 2024,11, 4070-4076
- 2023
-
Asymmetric Gold(I)-Catalyzed Prins-Type Cyclization for the Construction of Tricyclic Scaffolds
A. Dupeux, E. Gentilini, V. Michelet, J. Org. Chem. 2023, 88, 9439–9446

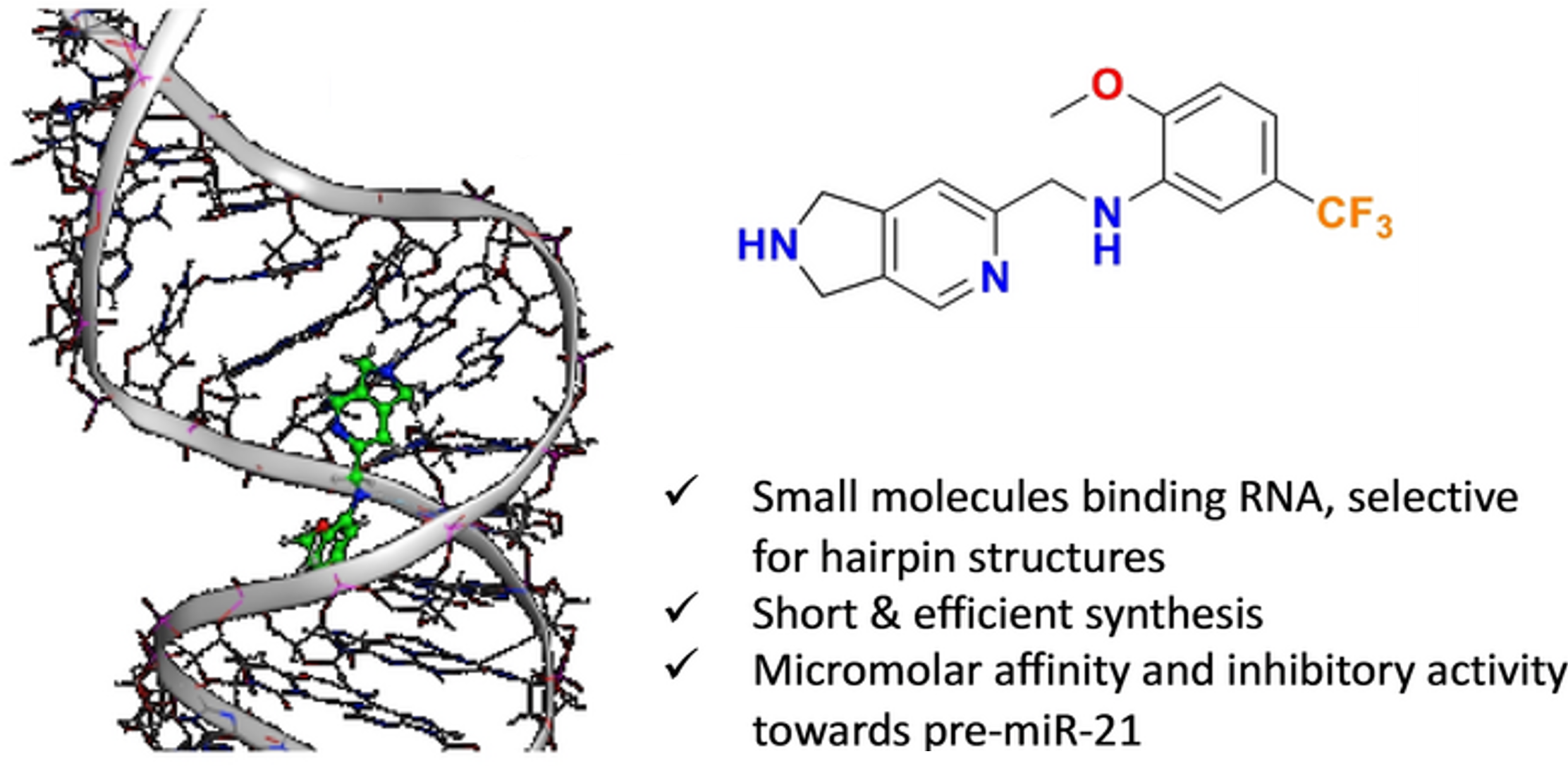
De-Novo Design of pre-miR-21 Maturation Inhibitors: Synthesis and Activity Assessment
I. Shcheholeva, D. Fernández-Remacha, R. Estrada-Tejedor, M. Duca, V. Michelet, Chem. Eur. J. 2023, 29, e202300825
Gold-Catalyzed One-Pot Cycloisomerization/Nucleophilic Addition/Rearrangement of Acenaphthylene Carbaldehyde Derivatives
A. Truchon, A. Dupeux, S. Olivero, V. Michelet, Adv. Synth. Catal. 2023, 365, 2006-2012

Gold(I)-Catalyzed Alkynylation of N,O-Acetals: An Access to Alkynylated Saturated N-heterocycles
R. Melot, S. Olivero, V. Michelet, Adv. Synth. Catal. 2023, 365, 496-501

Chiral 1,4-Oxazino[4,3-a]indoles as a Challenging Scaffold: Syntheses and Properties
A. Dupeux, V. Michelet, Synthesis 2022, 55, 240-245

- 2022
-
Phosphorous NMR Analysis and Activity of Chiral BINAP-Silver Complexes
K. Plevová, L. J. Prieto Pabon, M. Gaysinski, S. Poulain-Martini, V. Michelet, ChemPlusChem 2022, 87, e202200217

Transition Metal-Catalyzed Rearrangement and Cycloisomerization Reactions Toward Hedonic Materials
P. Martinaux, R. Laher, C. Marin, V. Michelet, Isr. J. Chem. 2023, 63, e202200047
Dedicated to Professor Alois Fürstner for his pioneer work on π-acid catalysis

Coinage Metal-Catalyzed Asymmetric Reactions of ortho-Alkynylaryl and Heteroaryl Aldehydes and Ketones
R. Melot, V. Michelet, Molecules 2022, 27, 6970-6984
In honor of Professor Henri B. Kagan
Use of Limonene Epoxides and Derivatives as Promising Monomers for Biobased Polymers
E. Louisy, V. Khodyrieva, S. Olivero, V. Michelet, A. Mija, ChemPlusChem 2022, 87, e202200190

On the Influence of the cis/trans Stereochemistry of Limonene Oxides toward the Synthesis of Biobased Thermosets by Crosslinking with Anhydrides
E. Louisy, S. Olivero, V. Michelet, A. Mija, ACS Sustainable Chem. Eng. 2022, 10, 7169-7179

Patent :
Polymères contenant des unités de répétition avec plusieurs motifs sulfonates métalliques ou organiques, leurs procédés de préparation et leurs utilisations comme antibactériens, fongicides, antiviraux et catalyseurs
J.-R. Desmurs, E. Dunach Clinet, S. Olivero, A. Adao, P. Knauth, V. Michelet, J. Cossy, FR2008940 et PCT/EP2021/074349, 2022Book chapter :
Gold catalysed C-C bond formation through C-H functionalization
R. Melot, C. Praveen, V. Michelet, Handbook of CH-Functionalization (CHF), Maiti, D.; Wiley-VCH, 2022

- 2021
-
Gold-Catalyzed Regioselective Oxyfluorination/Oxydifluorination vs. Diketonization of Phthalimido-Protected Propargylamines with Selectfluor
V. Marsicano, A. Arcadi, V. Michelet, Eur. J. Org. Chem. 2022, e202101524

Gold-catalyzed reactions towards diversity: from simple substrates to functionalized carbo- and heterocycles
V. Michelet, Chem. Rec. 2021, 21, 3884-3896

La catalyse à l’or en parfumerie
R. Laher, C. Marin, V. Michelet, l'Actualité Chimique 2021, 467, 55-56

Gold-Catalyzed Domino Cycloisomerization/Alkoxylation: an Entry to 3,4-Dihydro-1H-[1,4]oxazino[4,3-a]indole
A. Dupeux, V. Michelet, J. Org. Chem. 2021, 86, 17738-17747

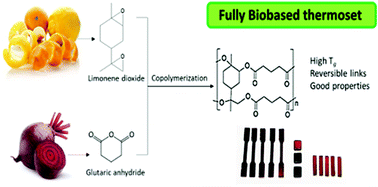
Limonene dioxide as building blocks for 100% bio-based thermosets
A. Mija, E. Louisy, S. Lachegur, V. Khodyrieva, P. Martinaux, S. Olivero, V. Michelet, Green Chem. 2021, 23, 9855-9859
Oxygen-tethered 1,6-enynes and [4.1.0]-Bicyclic ether skeletons as hedonic materials for the fragrance industry
R. Laher, E. Gentilini, C. Marin, V. Michelet, Synthesis 2021, 53, 4020-4029

An Anionic, Chelating C(sp3)/NHC ligand from the Combination of an N-heterobicyclic Carbene and Barbituric Heterocycle
I. Benaissa, K. Gajda, L. Vendier, N. Lugan, A. Kajetanowicz, K. Grela, V. Michelet, V. César, S. Bastin, Organometallics 2021, 40, 3223-3234

Catalytic Gold Chemistry: From Simple Salts to Complexes for Regioselective C-H Bond Functionalization
C. Praveen, A. Dupeux, V. Michelet, Chem. Eur. J. 2021, 27, 10495-10532

Indium-Catalyzed Cycloisomerization of 1,6-Cyclohexenylalkynes
V. Davenel, C. Puteaux, C. Nisole, F. Fontaine-Vive, J.-M. Fourquez, V. Michelet, Catalysts 2021, 11, 546
Special Issue - Recent Advances in Organometallic Chemistry and Catalysis

Silver-catalyzed tandem cycloisomerization/hydroarylation reactions and mechanistic investigations for an efficient access to 1,2-dihydroisoquinolines
M. De Abreu, Y. Tang, E. Brachet, M. Selkti, V. Michelet, P. Belmont, Org. Biomol. Chem. 2021, 19, 1037-1046
Energetics and Structures of Adducts of JohnPhos(Au+), PPh3(Au+), and IPr(Au+) with Organic Substrates: A Mass Spectrometry and DFT Study
C. Iacobucci, L. Massi, E. Duñach, P. Burk, J.-F. Gal, Organometallics 2021, 40, 11, 1642-1653

Chemical Characterization and Antibacterial Efficacy of Essential Oils of Three Lamiaceae Species Growing in Cameroon
H. G. Mbuntcha, H. P. Dogmo Fogang, V. Woguem, P. Sonkoue, P. Kenfack, K. Djeuga, C. Schippa, E. Duñach, J. A. Seukep, S. T. Lacmata, T. Fonkou, L. A. Tapondjou, H. M. Womeni, Journal of Diseases and Medicinal Plants 2021, 7, 14-21
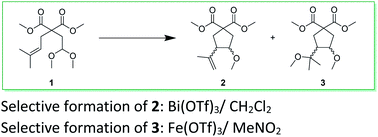
Bi(OTf)3-catalysed intramolecular cyclisation of unsaturated acetals
R. Saget, P. Jaunky, E. Duñach, RSC Adv. 2021, 11, 21066-21072
- 2020
-
Gold-Catalyzed Cycloisomerization of 1,6-Cyclohexenylalkyne: An Efficient Entry to Bicyclo[3.2.1]oct-2-ene and Bicyclo[3.3.1]nonadiene
V. Davenel, C. Nisole, F. Fontaine-Vive, J.-M. Fourquez, A.-M. Chollet, V. Michelet, J. Org. Chem. 2020, 85, 12657-12669

Experimental and Computational Evidences on Gold-Catalyzed Regioselective Hydration of Phthalimido-Protected Propargylamines: An Entry to β-Amino Ketones
V. Marsicano, A. Arcadi, M. Aschi, V. Michelet, Org. Biomol. Chem. 2020, 18, 9438-9447
Ruthenium Metathesis: A Key Step To Access a New Cyclic Tetrasubstituted Olefin Platform
C. F. Heinrich, D. Durand, J. Starck, V. Michelet, Org. Lett. 2020, 22, 7064-7067

Silver-catalyzed intramolecular [4 + 2] cycloaddition reaction of amide-1,6-enynes
X. Chen, F. Fontaine-Vive, S. Poulain-Martini, V. Michelet, Catal. Commun. 2020, 147, 106117

Heterogeneous catalysis for the tandem cyclisation of unsaturated alcohols
L. Seijo, P. Ondet, S. Olivero, E. Duñach, New J. Chem. 2020, 44, 10479-10483
When Gold Meets Perfumes: Synthesis of Olfactive Compounds via Gold-Catalyzed Cycloisomerization Reactions
R. Laher, C. Marin, V. Michelet, Org. Lett. 2020, 22, 4058-4062

Bi(OTf)3-catalysed regioselective arylation of Morita-Baylis-Hillman type allylic electrophiles
A. Omrania, F. Rezgui, E. Dunach, S. Poulain-Martini, Tetrahedron Letters 2020, 61, 151758

Room temperature palladium-catalyzed hydroarylation of ynamides in water
A. Di Nicola, V. Marsicano, A. Arcadi, V. Michelet, Tetrahedron Letters 2020, 61, 151725

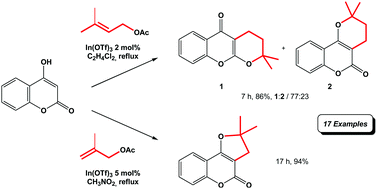
In(OTf)3-Catalysed Easy Access to Dihydropyranocoumarin and Dihydropyranochromone Derivatives
N. Boufroua, E. Dunach, F. Fontaine-Vive, S. Achouche-Bouzroura, S. Poulain-Martini, New J. Chem. 2020, 44, 6042-6052
Synthesis of functionalized dihydroimidazo[1,2-A]pyridines and 4-thiazolidinone derivatives from maleimide, as new class of antimicrobial agents
L. Salhi, S. Achouche-Bouzroura, R. Nechak, B. Nedjar-Kolli, C. Rabia, H. Merazig, S. Poulain-Martini, E. Duñach, Synth. Commun. 2019, 50, 2019, 412-422

- 2019
- Juniperanol: First Total Synthesis and Evaluation in type 2 Diabetes Disease
A. Carrër, S. Turban, N. Provost, A. Caliez, G. Lamarche, G. Zanirato, M. Beucher, C. Pean, O. Mirguet, F. Perron-Sierra, V. Michelet, Bioorg. Chem. 2019, 92, 103243

Cyclisation Reactions Involving Alkyl Enol Ethers
L. Lempenauer, G. Lemière, E. Duñach. Adv. Synth. Catal. 2019, 361, 5284-5304

A mild and regioselective synthesis of α-fluoroketones via gold and Selectfluor partnership
X. Chen, S. Martini, V. Michelet, Adv. Synth. Catal. 2019, 361, 3612-3618

An Original L-shape, Tunable N-Heterocyclic Carbene Platform for Efficient Gold(I) Catalysis
Y. Tang, I. Benaissa, M. Huynh, L. Vendier, N. Lugan, S. Bastin, P. Belmont, V. César, V. Michelet, Angew. Chem. Int. Ed. 2019, 58, 7977-7981

The electron-poor vinylcyclopropanes in organometallic catalysis: interesting skeletons with various reactivities
M. Laugeois, M. Vitale, V. Michelet, V. Ratovelomanana-Vidal, Transit. Met. Chem. 2019, 19, 1-35
Synthesis and olfactory evaluation of allylic α-quaternary ether ketones
L. Lempenauer, A. Soupart, E. Duñach, G. Lemière, Flavour Fragr. J. 2019, 34, 90-103
Synthesis and olfactory evaluation of allylic α-quaternary thioether ketones
L. Lempenauer, T. Appleson, G. Lemière, E. Duñach, Flavour Fragr. J. 2019, 34, 36-42
Direct and selective C-H carbamoylation of (hetero)aromatics with TMSOTf activated carbamoyl chloride
A. Uehara, S. Olivero, B. Michelet, A. Martin-Mingot, S. Thibaudeau, E. Duñach, Eur. J. Org. Chem. 2019, 46-49

Quality control of a functionalized polymer catalyst by energy dispersive X-ray spectrometry (EDX/EDS)
D. Hector, S. Olivero, F. Orange, E. Duñach, J.-F. Gal, Anal. Chem. 2019, 91, 1773-1778

- Older scientific productions

ICN