Enivronmental Radiochemistry
Understand the transfer mechanisms in the environment, define the chemical forms involved and understand their reactivity in a natural compartment (lake water, sea water, plants, soil, etc.).
Environmental radiochemistry
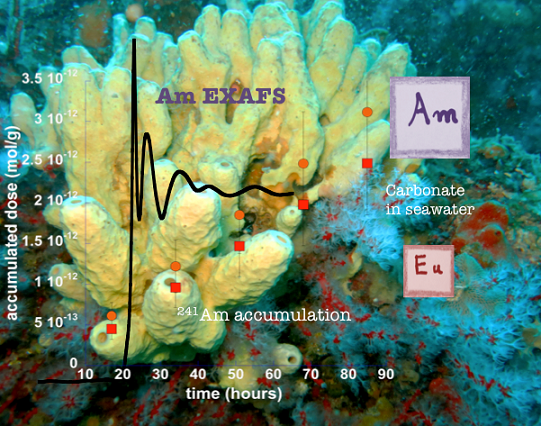
The dispersion of radionuclides (RN) in the environment, the biotope and therefore ultimately towards humans is one of the major concerns of civil society, as the Fukushima accident in March 2011 showed very well. This dispersion can be of anthropogenic origin (uranium mining activity, nuclear weapons or terrorism, civilian nuclear accidents) or less visibly natural (uranium-rich or thorium-rich soils). To this must also be added activities related to nuclear medicine. Speciation is data that is often difficult to obtain because NR concentrations in natural environments are extremely low (eg: 10-17 M in the Mediterranean for Pu-239,240, therefore much lower than ppb), but it is essential data. if we want to correctly understand the dispersion of NRs in an accident situation. In addition, the toxicity and bioavailability of elements often depend on their speciation, hence the capital importance of this factor on the toxicological impact. The modes of transfer and migration of RNs also depend on physico-chemical factors that are often poorly understood and among which we can cite pH, ionic strength, potential, etc. Such mechanisms are therefore extremely complex and our approach therefore goes beyond the environmental sciences and radioecology alone, it aims to explore the chemical (molecular) mechanisms underlying the phenomena of transport of low doses in the natural environment.