Theme #1: Synthetic methodology, catalysis and continuous flow chemistry
Nanocausys project (2012-2016)
Funded by ANR CD2I
The project Nanocausys (Nanostructured catalysts in unconventional systems), involving 5 French academic laboratories (ICN, ISM, ICMCB, ICCF, LOF) and the European chemical company Solvay (via E2P2L), was funded by ANR program “Sustainable Chemistry, Industry, Innovation”. This project was aimed at demonstrating the feasibility of heterogeneous catalysis in continuous reactors for industrial applications. We have chosen an integrated approach taking into account matrix, catalysts and reactor designs through the combined expertise of scientists from material science, catalysis and biocatalysis, microfluidics and reactor engineering. Nanostructured catalysts will be used in 3 categories of coupling reactions, implemented in continuous reactors, and eventually applied industrially in the synthesis of biosourced ingredients.
ICN : Institut de Chimie de Nice (U. Cote d'Azur - CNRS)
ISM : Institut des Sciences Moléculaires (U. Bordeaux - CNRS)
IICMCB : Institut de Chimie de la Matière Condensée de Bordeaux (U. Bordeaux - CNRS)
ICCF : Institut de Chimie de Clermont-Ferrand (U. Clermont-Ferrand - CNRS)
LOF : Laboratory of the Future (U. Bordeaux - CNRS - Solvay)
E2P2L : Eco-Efficient Products & Processes Laboratory (CNRS - Solvay - ENS Lyon - East China Normal University)
Hydroalkoxylation of olefins
Collab. with Dr Mathieu Pucheault - ISM Bordeaux
The transfer of catalytic hydroalkoxylation reactions of olefins from homogeneous to heterogeneous conditions has been studied using Montmorillonite (MMT) doped with metal cations. In the case of intramolecular reactions, 38-99% yields of cyclic ethers have been obtained using Fe-MMT and Bi-MMT both in nitromethane and dimethylcarbonate (DMC) compared with other supported metals salts or metal nanoparticles. In the case of more challenging intermolecular reactions, conversions up to 72% and yields up to 54% were obtained with metal-doped MMT as well, such as Fe-, Bi-, and Ti-MMT. These catalytic systems were shown to be highly recyclable and amenable to flow chemistry.
I. Notar Francesco, B. Cacciuttolo, M. Pucheault, S. Antoniotti. Simple metal salts supported on Montmorillonite as recyclable catalysts for intramolecular hydroalkoxylation of double bonds in conventional and VOC exempt solvents. Green Chem. 2015, 17 (2), 837 - 841. Link.
I. Notar Francesco, B. Cacciuttolo, O. Pascu, C. Aymonier, M. Pucheault, S. Antoniotti. Simple salts of abondant metals (Fe, Bi, and Ti) supported on Montmorillonite as efficient and recyclable catalysts for regioselective intramolecular and intermolecular hydroalkoxylation reactions of double bonds and tandem processes. RSC Advances, 2016, 6, 19807 - 19818. Link.
Multicatalytic processes based on Au NP catalysis (2013-present)
The project is aimed at developing novel atom- and step-economic reactions catalysed by metal nanoparticles. The philosophy is to merge homogenous and heterogeneous catalysis to get the advantages of both (basically selectivity and recyclability).
A current project we are working on deals with the setup of multicatalytic reactions, either fully orthogonal, sequential one-pot or in continuous flow. High level of atom- and step-economy can be achieved by these approaches.
Minireview article: I. Notar Francesco, F. Fontaine-Vive, S. Antoniotti. Synergies in the catalytic activity of bimetallic nanoparticles and new synthetic methods for the preparation of fine chemicals. ChemCatChem, 2014, 6, 10, 2784-2791. Link.
Book chapter: P. D. Giorgi, S. Antoniotti. Catalytic tandem reactions triggered by the introduction of a carbonyl function. Advances in Organic Synthesis, Ed. Atta-ur-Rahman, 2018, 8, 1-31 (Invitation). Link.
Novel radical tandem 1,6-enynes thioacylation / cyclisation catalysed by Au-Pd nanoparticles
Collab. with Prof. Graham J. Hutchings - Cardiff Catalysis Institute
We studied the reactivity of 1,6-enynes with thioacetic acid (AcSH) under either thermal conditions or in the presence of catalytic amounts of supported Au or Au-Pd nanoparticles (NPs) under mild conditions. The 1,6-enynes undergo a tandem thioacylation / cyclisation to original cyclic products featuring either a homoallylic thioester function or an enol thioester function depending on the substrate topology. Interestingly, the former process was found more efficient when performed in the presence of Au-Pd NPs while the latter process can be efficiently carried out under thermal conditions (100 °C). The reaction proceeds by a radical mechanism and the presence of precious metal NPs seems to stabilise the formation of free radical intermediates, as supported by experimental and theoretical results.
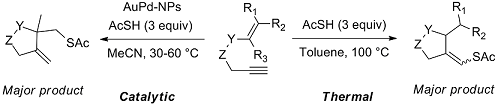
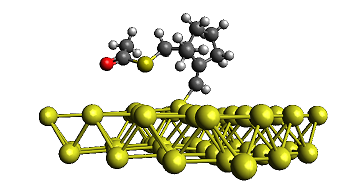
I. Notar Francesco, J. Giauffret, F. Fontaine-Vive, J. K. Edwards, C. J. Kiely, G. J. Hutchings, S. Antoniotti. Novel radical tandem 1,6-enynes thioacylation / cyclisation : Au-Pd nanoparticles catalysis versus thermal activation as a function of the substrate specificity. Tetrahedron, 2014, 70, 51, 9635–9643. Link.
Multicatalytic reactions en route to the one-pot total synthesis of complex molecules
A first example was recently published with an easy access to chromene and 1,2-dihydroquinoline derivatives from simple substrates catalysed by Au NPs and simple bases.
P. D. Giorgi, P. J. Miedziak, J. K. Edwards, G. J. Hutchings, S. Antoniotti. Bicatalytic multistep reactions en route to the one-pot total synthesis of complex molecules : easy access to chromene and 1,2-dihydroquinoline derivatives from simple substrates. ChemCatChem 2017, 9, 1, 70-75.. Cover picture. Link. Highlighted by Wiley in Hot topics : sustainable chemistry.
P. D. Giorgi, N. Elizarov, S. Antoniotti. Selective oxidation of activated alcohols by supported gold nanoparticles under an atmospheric pressure of O2 : batch and continuous flow studies. ChemCatChem 2017, 9, 10, 1830-1836. Link.
N. Elizarov, P. D. Giorgi, A. Yeromina, S. Antoniotti. Complex molecules synthesis made easy. Proceedings of the 1st UCA days 2018. Link.
We further developed an efficient access to original cannabinoids by combining different types of solid catalyst in continuous flow: P. D. Giorgi, V. Liautard, M. Pucheault, S. Antoniotti. Biomimetic Cannabinoid Synthesis Revisited: Batch and Flow All‐Catalytic Synthesis of (±)‐ortho‐Tetrahydrocannabinols and Analogues from Natural Feedstocks. Eur. J. Org. Chem. 2018, 11, 1307-1311. Cover picture. Link.
With peculiar structural features at the surface of small metal nanoparticles, some discrete sites can display catalytic behaviour similar to what could be observed with mononuclear metal catalysts in solution. We have studied the transfer of two catalytic tandem reactions from homogeneous to heterogeneous conditions. Tandem cyclisation/reduction of ortho-alkynyl benzaldehyde derivatives was successfully achieved with Au nanoparticles over TiO2 (Au NPs/TiO2) in the presence of Hantzsch ester with 45-98% yields for 15 examples (average yield: 70.4%). Similarly, tandem cyclisation/hydroalkoxylation of ortho-alkynyl benzaldehyde derivatives was successfully achieved with Au NPs/TiO2 in methanol or other alcohols with 62-96% yields for 17 examples (average yield: 84.9%). The application potential of this catalytic system was demonstrated with the total synthesis of a bioactive isochromene derivative featuring one of the developed reactions as the key step and the implementation of the tandem cyclisation/hydroalkoxylation in a continuous flow reactor, scaling up the reaction by a factor of 10 without loss of efficiency.
K. Plevova, V. Michelet, S. Antoniotti. Emulation of Transition Metal-based Catalytic Systems by Small Gold Nanoparticles. Tandem Cyclisation/Reduction and Cyclisation/Hydroalkoxylation Reactions. Commun. Chem. 2024, 7, 248. Nature (OPEN ACCESS).
Nanocatalysis with bimetallic catalysts
Theme #2: Biocatalysis and Innovative fragrance ingredients
Review articles:
S. Antoniotti. Tuning of essential oils properties by enzymatic treatment : Towards sustainable processes for the generation of new fragrant ingredients. Molecules, 2014, 19(7), 9203-9214. (Invitation) OPEN ACCESS.
M. Lecourt, S. Antoniotti. Chemistry, sustainability and naturality of perfumery biotech ingredients. ChemSusChem, 2021, 13, 21, 5600-5610. Link.
Essenzyme project (2014-2018)

Labelised by cluster PASS
Funded by APRF CR PACA
The project Essenzyme is a collaborative project between our group and two French companies, aimed at developping mild enzymatic protocols for the tuning of essential oils properties by modification of their chemical composition. It is divided in several sub-projects, one for the design of novel fragrance ingredients, and the second for the production of detoxified essential oils.
Natural vetiver acetate project (2015-2019)
Funded by SATT sud-est
Development of an industrial process for the manufacture of natural vetiveryl acetate for the perfume industry. European patent EP3303533 (A1).
I. Notar Francesco, J.-J. Filippi, S. Antoniotti. Sustainable manufacture of a valuable fragrance ingredient : Lipase-catalysed acylation of vetiver essential oil and chemoselectivity between sesquiterpene alcohols. ChemPlusChem, 2017, 82, 3, 407-415. DOI : 10.1002/cplu.201600617. Link. Highlighted in the Wiley Hot topics : biocatalysis.
Natural atranol-free oakmoss extracts (2014-2019)
Funded by SATT sud-est.
Development of an enzymatic process for the removal of atranol and related toxic compounds from oakmoss extracts. European patent pending.
H. Bouges, A. Monchot, S. Antoniotti. Enzyme-catalysed conversion of atranol and derivatives into dimeric hydrosoluble materials. Application to the preparation of a low-atranol oakmoss absolute. Cosmetics 2018, 5(4), 69. Link.
H. Bouges, K. Calabro, O. P. Thomas, S. Antoniotti. Unusual polycyclic fused product by oxidative enzymatic dimerisation of 5-methylpyrogallol catalyzed by horseradish peroxidase/H2O2. Molecules 2018, 23(10), 2619. Link.
Discovery and total synthesis of fragrance ingredients by innovative technologies (2019-2022)
Funded by ANRT and Alysophil (CIFRE). PhD thesis of Laure Gilles. Co-advisory: Dr. N. Baldovini.
L. Gilles, S. Antoniotti. Chemical and olfactory analysis of the volatile fraction of Ocimum gratissimum concrete from Madagascar. Chem. Biodiv. 2023, e202300252. Wiley (OPEN ACCESS). HAL.
L. Gilles, S. Antoniotti. Spirocyclic compounds in fragrance chemistry: synthesis and olfactory properties. ChemPlusChem, 2022, 87, 11. sous presse. Link.
L. Gilles, S. Antoniotti. Chimie durable et parfumerie. Nez, La revue 2020, link.
E. Tempio, A. Ravez, D. Lach, M. Kapkowski, K. Plevova, L. Gilles, J. Polanski, S. Antoniotti. Iron(III) Chloride-catalysed acetalisation of α,β-unsaturated carbonyl compounds towards odorant ketals. Tetrahedron 2023, 149, 133734. Elsevier.
Optimisation of the properties of natural complex substances used in perfumery by enzymatic treatments (2019-2022)
Funded by IFF-LMR and Région PACA. PhD thesis of Mathilde Lecourt.
Labelised by cluster PASS.
M. Lecourt, G. Chietera, B. Blerot, F. Jullien, S. Antoniotti. From biosynthesis in plants to post-biosynthetic enzymatic conversion. Generation of odor-impact rose oxides from citronellol-rich essential oils. Botany Lett., 2023, 70, 1, 15-27. Lien vers le site de l'éditeur. Lien vers l'archive ouverte HAL.
M. Lecourt, C. Giorgiana, B. Blerot, S. Antoniotti. Laccase-catalyzed oxidation of allylbenzene derivatives: towards a green equivalent of ozonolysis. Molecules, 2021, 26, 19, 6053. Lien vers le site de l'éditeur. Lien vers l'archive ouverte HAL.
M. Lecourt, S. Antoniotti. Les biotechnologies blanches au service de la parfumerie et les nouveaux ingrédients biotech. Nez, La revue 2021, Lien vers le site de Nez, la revue.
M. Lecourt, S. Antoniotti. Chemistry, sustainability and naturality of perfumery biotech ingredients. ChemSusChem, 2021, 13, 21, 5600-5610. Lien vers le site de l'éditeur. Lien vers l'archive ouverte HAL.
M. Lecourt, G. Chietera, B. Blerot, F. Jullien, S. Antoniotti. From biosynthesis in plants to post-biosynthetic enzymatic conversion. Generation of odor-impact rose oxides from citronellol-rich essential oils. Botany Lett. 2023, 70, 1, 15-27. Taylor & Francis.
Theme #3: Analytical sciences and authenticity of natural ingredients for perfumery
Assessment of the naturally of essential oils by innovative approaches (2019-2022)
Funded by Pharmapur and IDEX UCAJEDI. PhD thesis of Marissa Pierson.
M. Pierson, X. Fernandez, S. Antoniotti. Study of the type and magnitude of cases of non-compliance and adulteration in neroli, mandarin and bergamot essential oils purchased on-line and vulnerability of end-users. Sci. Rep. 2021, 11, Article number: 11096. Link.
Model study of the phenomenon of migration of contaminants from plastic containers to essential oils (2018-2019)
Funded by ERINI and IDEX UCAJEDI. Master thesis of Bin Li and Jihene Ayari.
Typicity of Provence rosé wines by analytical and sensory approaches (2021-2024)
Labelised by Pole Innov'alliance
Funded by Région Sud PACA and Centre du Rosé. PhD thesis of Charlotte Richard. Co-advisory: X. Fernandez.
Perfumes comparison by combination of machine learning and analytical chemistry (2022-2025)
Funded by ANRT and Perfumist (CIFRE). PhD thesis of Jean-Baptiste Coffin. Co-advisory with Sebastien Fiorucci.
Méthodes de dosage des polluants et substances réglementées émis et transformés par les parfums d’ambiance dans les environnements intérieurs (2022-2025)
Funded by ANRT and société (CIFRE). PhD thesis of someone. Co-advisory with X. Fernandez.
GDRO3 : French transdisciplinary research network on odorants, odours and olfaction https://gdro3.wordpress.com/


